63+ calculate the amount of heat required to raise the temperature
Calculate the amount of heat required to raise. Lets assume that the perfect temperature.

The Quantity Of Heat The Thermal Energy Lost Or Gained By Objects Is Called Heat One Calorie Cal Is The Quantity Of Heat Required To Change The Temperature Ppt Video Online
Web The formula used by this calculator to determine the heat transferred from the heat capacity and change in temperature is.

. Q mCΔT where Q is the amount of heat. Web The Specific Heat c of water is 4186 joulegram C. How much heat is required to warm 130 kg sand from 240 C.
Web Find the amount of heat required to convert it into the water. Web Calculate the amount of heat needed to melt 130. Web The heat specific calculator helps to calculate the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius C.
The mass of gold is 600g 600 g. 0 g of aluminum from 3 5 o C to 5 5 o C. Amount of heat Mass Heat Capacity Temperature Change Heat capacity of Water 42 JgC Amount of heat 64 42 69 - 32.
Identify the mass and the specific heat capacity of the substance. There are two sig figs in the given. Determine how much energy you need to heat the water.
The specific heat capacity of gold is 0129Jg C 0129 J g C. It is the amount of heat. Q C ΔT.
Web We have mass in grams and temperatures in degrees celsius so we can look up the specific heat constant of water using in those given units. Web Calculate the amount of heat required to raise the temperature of 782 g of water from 100C to 320C. Round your answer to 3 significant digits.
Web The amount of heat required to raise the temperature of water sample can be calculated using the formula. So Q 76g 4186 joulegram C 62C - 31C or Q 9862216 joules. C Heat capacity.
Web How do i calculate the amount of heat required to raise the temperature of an 89 sample of water from 40 to 60. Web A copper vessel has mass 1 5 0 g and the specific heat capacity of copper is 4 1 0 J k g 1 K 1. How much heat energy will be required to increase the temperature of the.
The specific heat of water is 4184 Jg C. G of solid acetic acid HCH3CO₂ and bring it to a temperature of 643 C. Web How much heat in Joules is required to raise the temperature of 340 kg of water from 15 C to 95 C.
A Explain why the energy. Web Calculate the number of k J necessary to raise the temperature of 6 0. Molar heat capacity of A l is 2 4 J m o l 1 K 1.
From a reference table. Web The heat capacity CT of a substance is the amount of energy in joules required to raise the temperature of 1g by 1 degrees C at temperature T.

A Bond Energy Bond Order And Populations Relationship Journal Of Chemical Theory And Computation
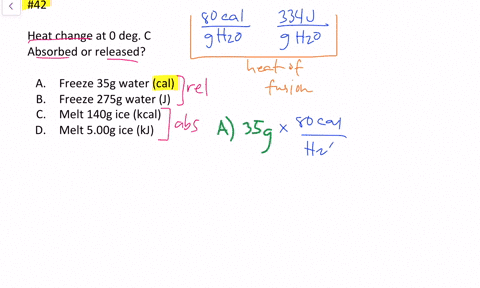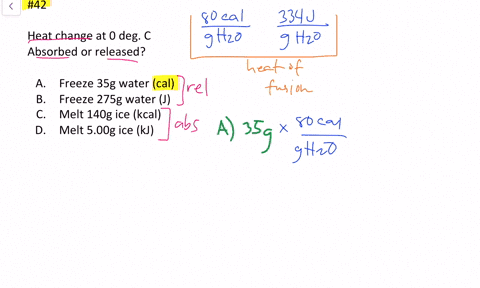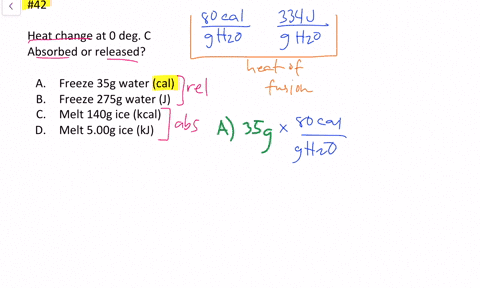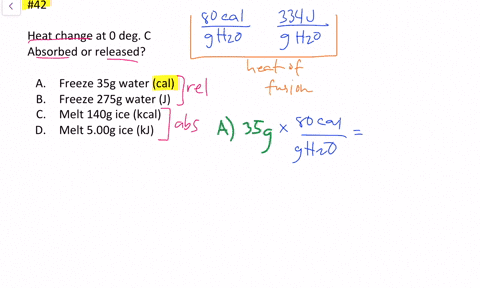
Solved Calculate The Heat Change At 0 C For Each Of The Following Problems Indicate Whether Heat Was Absorbed Or Released A Calories To Melt 65 G Of Ice B Joules To Melt

Specific Heat Calculator

Thermostability Tunability And Tenacity Of Rna As Rubbery Anionic Polymeric Materials In Nanotechnology And Nanomedicine Specific Cancer Targeting With Undetectable Toxicity Chemical Reviews

Find The Amount Of Heat Required To Raise The Temperature From 23 C To 50 C Mass Of Water Is Brainly In

Temperature Change And Heat Capacity Fundamentals Of Heat Light Sound

A Vessel Of Negligible Heat Capacity Contains 40g Of Ice At 0 O C 8g Of Steam At 100 O C Is Passed Into The Ice To Melt It Find The Final

Design Of Water Treatment Systems Pdf Pdf Alkalinity Drag Physics

Which Of The Following Processes Requires The Most Energy Input Given Below Is The Properties Of The Water Propertyvalue Specific Heat 1 0 Cal G O Clatent Heat Of Fusion 80 Cal Glatent Heat Of Vaporization540
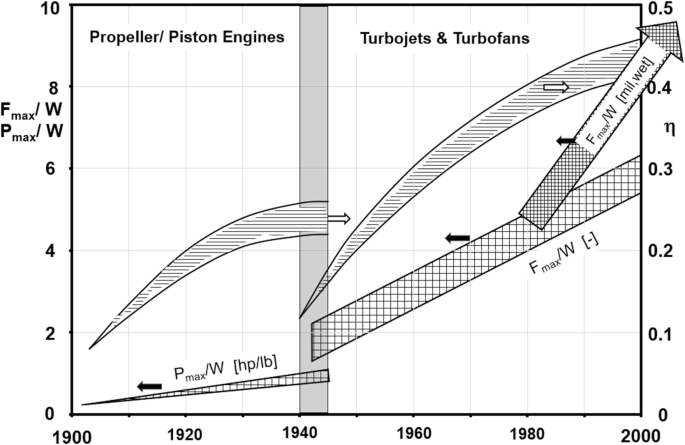
Survey Springerlink

Heat And The First Law Of Thermodynamics Ppt Download

Solved Write The Balanced Equations For The Reactions That Occurred When Hci Was Added To The Buffer Solution Hci Was Added T0 Water Type Thoughtful Response To The Following Question Be Sure

Solved O 932j Calculate The Heat Required To Raise The Chegg Com

Chem Ii Final Review Flashcards Quizlet

How Much Heat Energy Is Required To Raise The Temperature Of 8 Kg Water From 10 To 90 Quora
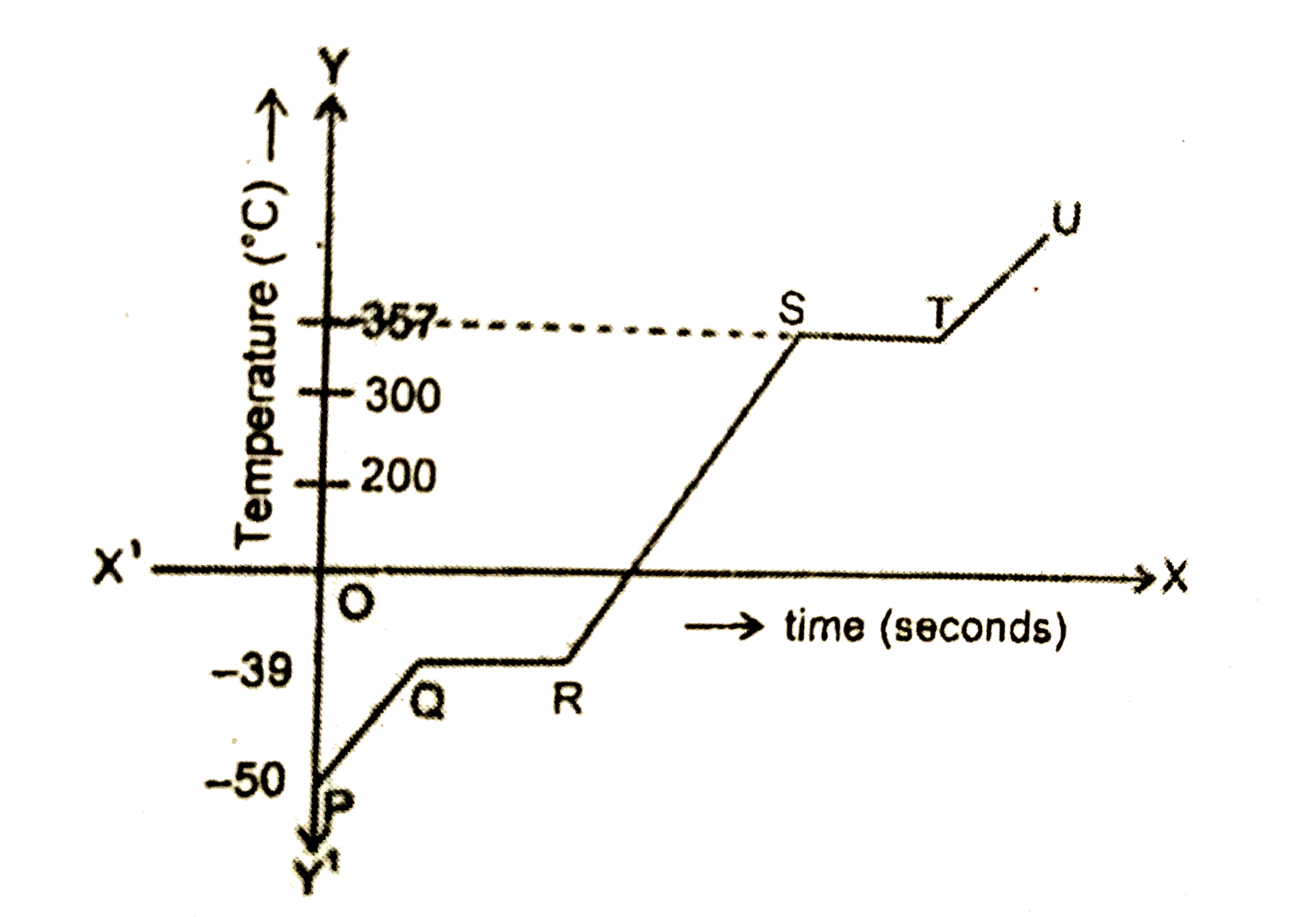
Calculate The Amount Of Heat Energy Required To Increase The Temperature Of 250 G Of Water From 27 C To 67 C Specific Heat Capacity Of Water Is 1 Cal G 1 C 1

August 2017 Skm Classes Bangalore Page 2